Part:BBa_K3717005
Endo-β-Galactosidase with strong promoter and RBS

Figure 1: Endo-β-galactosidase from 2014 Tuebingen with strong promoter + RBS
Construct Design
Endo-β-galactosidase an enzyme that catalyzes the cleavage of the A and B type blood antigens trisaccharides such that the remaining sugar can be classified as a H antigen, which the anti-A and anti-B antibodies are unable to recognize and thus does not elicit an immune response in the human body [1]. Thus, the enzyme can convert both A and B blood types to universal O type.
We obtained the amino acid sequence of the endo-β-galactosidase protein, derived from Clostridium perfringens, the iGEM DNA Repository Plate (BBa_K1483001), which served as our Open Reading Frame (ORF). We attached a strong promoter and strong ribosome binding site (RBS; BBa_K880005) upstream of the open reading frame (ORF). The composite gene was synthesized through DNA cloning.
Contributions

Figure 1 - Colony PCR check for strong promoter (K88) Endo-β-Galactosidase (Part:BBa_K3717005) using VF2 and VR primers. Confirms successful ligation as a band is produced at the expected size of 2752bp, as indicated by the red triangle.
We tested protein expression of the composite part BBa_K3717005 by transforming our plasmids into BL21 E. Coli cells. We grew cultures at 37°C overnight, diluted those cultures, and then grew to OD600 0.5~0.6 at 37°C. We then induced expression with 0.5 mM IPTG and allowed cultures to grow overnight at room temperature. We took samples pre-induction and post-induction and examined them by SDS-PAGE.
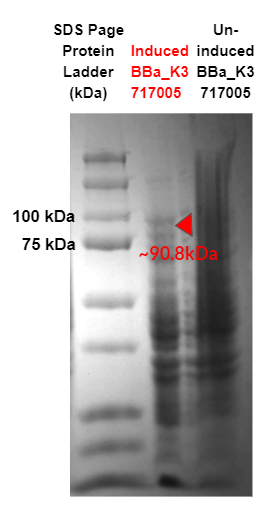
Figure 2 - SDS Page of cell lysate for each strain: strong promoter (K88) Endo-β-Galactosidase (Endo-β-gal) (Part:BBa_K3717005). Red triangles indicate expected size for Endo-β-gal (90.8 kDa). Sequences for target proteins were found to not contain a start codon and thus have no expression, as shown by the triangles.
Our SDS-page in Fig. 2 did not show any overexpression bands for the enzymes of interest. The results indicate that there were no target proteins at their expected band sizes: 90.8 kDa band for K88 promoter + Endo-β-gal in the induced sample. As the SDS page is of cell lysis samples, other bands present are due to innate proteins present in the bacteria cell.
Upon comparison of the amino acid sequence from Tuebingen’s parts with expected full sequences that were obtained from UniProt, we discovered that the enzyme sequences were missing the start codon (Fig. 3), which explained the non-expression of the proteins.
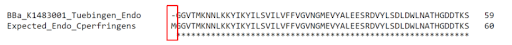
Figure 3 - Top sequence: First 59 amino acids of Team Tuebingen's 2014 Endo-β-gal part BBa_K1483001. Bottom sequence: First 60 amino acids of expected sequence for Endo-β-gal derived from Clostridium perfringens. Based on the alignment of the two sequences, Tuebingen's part is missing the first amino acid (start codon) of the Endo-β-gal protein.
References
1.Rahfeld, Peter, and Stephen G. Withers. “Toward Universal Donor Blood: Enzymatic Conversion of A and B to O Type.” Journal of Biological Chemistry, vol. 295, no. 2, Jan. 2020, pp. 325–34. DOI.org (Crossref), https://doi.org/10.1074/jbc.REV119.008164.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |
